By Kebba Ansu Manneh
Markieu Janneh Kaira, Executive Director of Medicines Control Agency, The Gambia has disclosed that investigations are on course to establish the companies found liable for the death of 66 Gambians children who suffered from Acute Kidney Injuries (AKI) will be prosecuted.
She made this disclosure in a telephone interview with this reporter where she said the Medicines Control Agency is not yet in the position to disclose the names of local importers of pharmaceutical products in The Gambia.
The Executive Director of Medicines Control Agency’s reaction following WHO alert over India-made cough syrups after deaths in The Gambia and warned they could be linked to the deaths of 66 children in The Gambia.
The syrups have been “potentially linked with acute kidney injuries and 66 deaths among children”, WHO said.
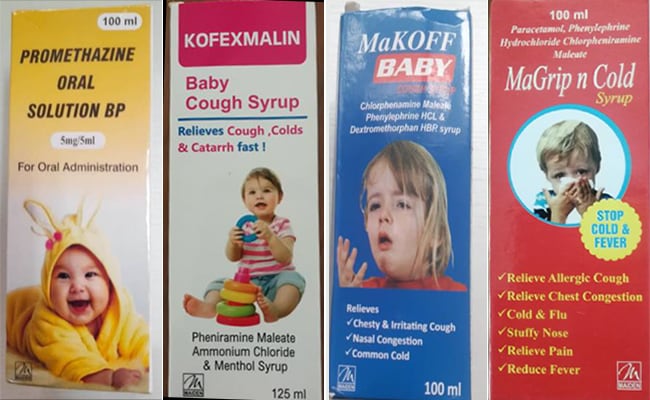
The global health organization has also called on other countries to be vigilant in the distribution of these syrups in their countries, naming the syrups as Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup that are allegedly manufacturer is Maiden Pharmaceuticals Limited in India.
“We are doing our findings and investigation regarding this issue and we are liaising with the police who are the right institution to do any prosecution. This is a legal matter, so if there is going to be any prosecution of any company involved the police will take that legal action,” she said.
MCA Executive Director declined to reveal the names of the local pharmaceutical companies that are partnering with the manufacturer of the Syrup that continues to cause havoc in the lives of children’s under five years, maintaining that after MCA is done with its findings police will proceed to press charges against any company that is liable for the crime.
When asked to shedlight on why Gambia is no more ordering its pharmaceutical products from International Dispensary Association (IDA), she also declined to comment, submitting that she has no idea why Gambia is no more procuring its pharmaceutical products from IDA in Amsterdam, Holland.
However, the Indian-based Maiden Pharmaceuticals Limited is said to be a repeat offender in faking pharmaceutical products and has been recognized by the WHO.